how many valence electrons does rb have|Rubidium : Tagatay The ability of one atom of an element to join another atom during the formation of a molecule is called valency(valence). The number of unpaired electrons in the last orbit of an element is the valency of that element. The electron configuration of rubidium . Tingnan ang higit pa Bob the Robber 3 Unblocked. For us, the third game of Bob the Robber is a masterpiece. In this adventure, Bob works for the intelligence service of the country. In other words, this is a call of duty. Bob puts on some cool gears and sneaks in the base of the harmful terrorist organizations. How cool gadgets?
PH0 · Valency of Rubidium
PH1 · Valences of the Chemical Elements
PH2 · Valence Electrons Chart for All Elements
PH3 · Rubidium valence electrons
PH4 · Rubidium Valence Electrons and Electron
PH5 · Rubidium Valence Electrons
PH6 · Rubidium (Rb)
PH7 · Rubidium
PH8 · How Many Valence Electrons Does Rubidium (Rb) Have?
PH9 · 10.6: Valence Electrons
Watch Sydney Sweeney's Breasts, Underwear scene on AZNude for free (1 minute and 12 seconds). Watch Sydney Sweeney's Breasts, Underwear scene on AZNude for free (1 minute and 12 seconds). . and usable. We have a free collection of nude celebs and movie sex scenes; which include naked celebs, lesbian, boobs, underwear and butt pics, hot .
how many valence electrons does rb have*******The elements that have 1, 2, or 3 electrons in the last shell donate the electrons in the last shell during bond formation. The elements that form bonds by donating electrons are called cations. The rubidium atom donates an electron of the last shell to form bonds and turns into a rubidium ion(Rb+). That . Tingnan ang higit paThe valence electrons have to be determined by following a few steps. The electron configuration is one of them. It is not . Tingnan ang higit pa
The ability of one atom of an element to join another atom during the formation of a molecule is called valency(valence). The number of unpaired electrons in the last orbit of an element is the valency of that element. The electron configuration of rubidium . Tingnan ang higit pa
The total number of electrons present in the valence shell of an atom is called valence electrons, and there is only one electron . Mar 23, 2023
How many valence electrons does Rubidium have? Rubidium basically belongs to the category of the Alkali metal group in the periodic table. It has the .
The atomic number of each element increases by one, reading from left to right. BlockElements are organised into blocks by the orbital type in which the outer electrons . Subscribed. 8. 784 views 1 year ago Electron Configuration. Valence electrons are those electrons present in the outermost shell of an atom. In this video, we will see two ways by which one. May 19, 2024
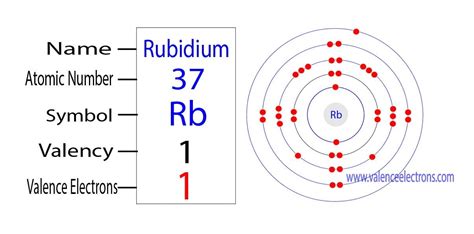
The information on this page is fact-checked. Rubidium valence electrons | Image: Learnool. Rubidium, classified as an alkali metal, possesses 1 valence electron. .Rubidium is a chemical element of the periodic table with chemical symbol Rb and atomic number 37 with an atomic weight of 85.4678 u and is classed as a alkali metal.
Example \(\PageIndex{1}\): Number of Valence Electrons. How many valence electrons are in one atom of each element? sulfur; helium; potassium; . Use the group numbers to determine the number of valence electrons. The Group number of a non-transition metal can be used to find the number of valence electrons in an atom of that element. The ones .how many valence electrons does rb have Rubidium How can you use the periodic table to find out how many valence electrons an element has? This article from Khan Academy explains the simple rules and patterns that can help you determine the number of electrons in the outermost shell of any atom. You will also learn why valence electrons are important for chemical bonding and reactivity.
How many valence electrons does boron have? Recognize that the second principal energy level consists of both the \(2s\) and the \(2p\) sublevels, and so the answer is three. B: 1s 2 2s 2 2p 1 (there are three electrons on the highest occupied energy level n=2)
How many valence electrons does Rb have? 1 -apex. What is Rb's number of protons? There is actually 37 Protons in Rb (Rubidium). Also there is the same number of protons as electrons. So 37 for .The electrons that determine valence – how an atom reacts chemically – are those with the highest energy.. For a main-group element, the valence electrons are defined as those electrons residing in the electronic shell of highest principal quantum number n. [1] Thus, the number of valence electrons that it may have depends on the electron .
This table of element valences includes the maximum valence and most common valence values in chemistry. Use this for reference with a periodic table. . While these are the most common valences, the real behavior of electrons is less simple. Remember an element's electron cloud will become more stable by filling, emptying, or . The valence electrons of each main-group element can be determined by the column in which it is located. (i.e., all group 1 elements have 1 valence electron, all group 2 elements have 2 valence electrons, skip the transition metals. then, all group 13 elements have 3 valence electrons, all group 14 elements have 4 valence electrons, . Final answer: Rubidium (Rb) has one valence electron, following the periodic trend where group 1 elements have one valence electron.This single valence electron in rubidium is located in the 5s orbital. The abbreviated electron configuration for . 1. Determine the total number of valence electrons in the molecule or ion. Each hydrogen atom (group 1) has one valence electron, carbon (group 14) has 4 valence electrons, and oxygen (group 16) has 6 valence electrons, for a total of [(2)(1) + 4 + 6] = 12 valence electrons. 2. Arrange the atoms to show specific connections.
Valence electrons are those electrons present in the outermost shell of an atom. In this video, we will see two ways by which one can determine the valence e.
Solution. Element A is located in Period 2, the 5th position in 2p-block.Before the electrons are placed in 2p subshell, the 2s subshell must be filled first. This means that A has two valence electrons in 2s (2s 2) and five valence electrons in 2p (2p 5).Answer: 2s 2 2p 5. It has 2 + 5 = 7 valence electrons.. Element B is located in Period 3, the 2nd . The number of valence electrons in an atom may have the same or different numerical value as its oxidation state. For example, a lithium atom has 1 valence electron and has an oxidation state of +1. In .
Khanmigo is now free for all US educators! Plan lessons, develop exit tickets, and so much more with our AI teaching assistant.

The presence of valence electrons can determine the element's chemical properties and whether it may bond with other elements: For a main group element, a valence electron can only be in the outermost electron shell. An atom with a closed shell of valence electrons (corresponding to an electron configuration \(s^2p^6\)) tends to be chemically .
how many valence electrons does rb haveThe presence of valence electrons can determine the element's chemical properties and whether it may bond with other elements: For a main group element, a valence electron can only be in the outermost electron shell. An atom with a closed shell of valence electrons (corresponding to an electron configuration \(s^2p^6\)) tends to be chemically .
Rubidium Group 18 elements (helium, neon, and argon are shown in Figure 2) have a full outer, or valence, shell. A full valence shell is the most stable electron configuration. Elements in other groups have partially filled valence shells and gain or lose electrons to achieve a stable electron configuration.
If you're seeing this message, it means we're having trouble loading external resources on our website. If you're behind a web filter, please make sure that the domains *.kastatic.org and .
How many valence electrons does a Rubidium atom have? Rubidium has 1 valence electrons. Rubidium has 37 electrons out of which 1 valence electrons are present in the 5s1 outer orbitals of atom.Elements whose atoms have the same number of valence electrons are grouped together in the Periodic Table.. Generally, elements in Groups 1, 2, and 13 to 17 tend to react to form a closed shell with a noble gas electron configuration ending in #ns^2 np^6#.. METALS. The most reactive metals are those from Groups 1 and 2.
Find the best hotels near Christchurch Casino, Christchurch City Centre from AU$96! Relax with our Price Promise & FREE cancellation on select hotels. Book now, pay later!
how many valence electrons does rb have|Rubidium